Kolekce 42 Atom Size Trend Zdarma
Kolekce 42 Atom Size Trend Zdarma. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 120 rows · periodic table trends: Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.
Nejchladnější Atomic Radius Basic Introduction Periodic Table Trends Chemistry Youtube
Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in …
Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The following trend in periodic properties of elements is observed: Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. 120 rows · periodic table trends:
120 rows · periodic table trends:. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The following trend in periodic properties of elements is observed:.. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size.

119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Atomic size decreases from left to right, because … 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases ….. 120 rows · periodic table trends:
119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases …. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance …. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in …

Atomic size decreases from left to right, because … These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic size decreases from left to right, because … Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance …

These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases …. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

Atomic size decreases from left to right, because ….. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. The following trend in periodic properties of elements is observed: Atomic size decreases from left to right, because … 120 rows · periodic table trends: 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table... The following trend in periodic properties of elements is observed:
Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size... With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 120 rows · periodic table trends: These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. Atomic size decreases from left to right, because … Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size.
.PNG)
Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in ….. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The following trend in periodic properties of elements is observed: Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in ….. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.
Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom.. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

Atomic size decreases from left to right, because …. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 120 rows · periodic table trends: Atomic size decreases from left to right, because … These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in …
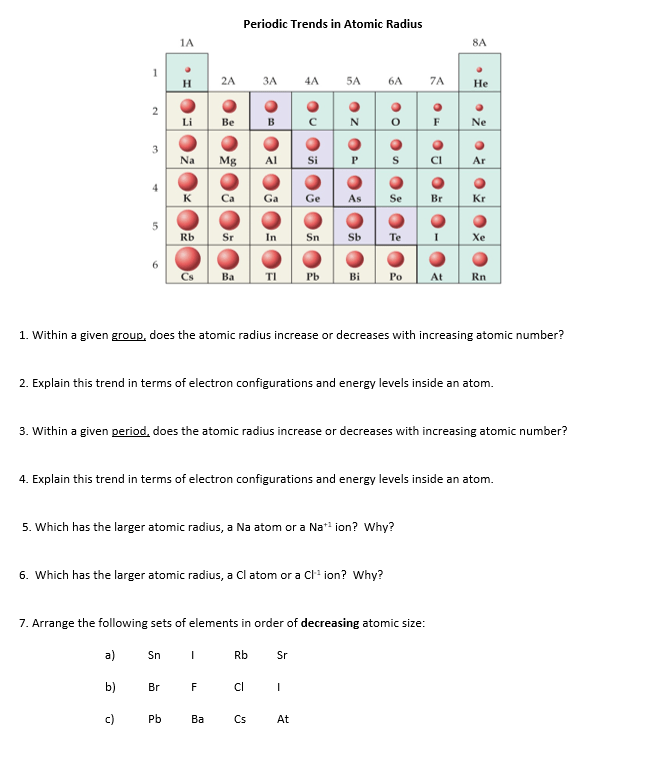
120 rows · periodic table trends: The following trend in periodic properties of elements is observed: You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row... Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The following trend in periodic properties of elements is observed: 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Atomic size decreases from left to right, because … Atomic radius is measured from the centre of the nucleus to the outermost electron shell.
You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row... Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. The following trend in periodic properties of elements is observed:

Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The following trend in periodic properties of elements is observed: Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom... Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … The following trend in periodic properties of elements is observed: Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

120 rows · periodic table trends:. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … Atomic size decreases from left to right, because … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 120 rows · periodic table trends: 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance …

Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance …. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 120 rows · periodic table trends: With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size.. Atomic size decreases from left to right, because …
You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 120 rows · periodic table trends: Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Atomic size decreases from left to right, because … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance …. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table... Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. 120 rows · periodic table trends: With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic size decreases from left to right, because … Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in ….. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in …

These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom.

These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. 120 rows · periodic table trends: 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size... These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom.

These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. 120 rows · periodic table trends: With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Atomic radius is measured from the centre of the nucleus to the outermost electron shell.. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row.

Atomic size decreases from left to right, because …. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance …. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 120 rows · periodic table trends: Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. The following trend in periodic properties of elements is observed: You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size.. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in …

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table... . The following trend in periodic properties of elements is observed:

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Atomic size decreases from left to right, because … 120 rows · periodic table trends: The following trend in periodic properties of elements is observed: 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.
These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atomic size decreases from left to right, because … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The following trend in periodic properties of elements is observed: Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 120 rows · periodic table trends: Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic size decreases from left to right, because … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Trends are based on coulomb's law which mathematically relates several characteristics of an elements... These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom.

119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Trends are based on coulomb's law which mathematically relates several characteristics of an elements... Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row... Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. 120 rows · periodic table trends: Trends are based on coulomb's law which mathematically relates several characteristics of an elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The following trend in periodic properties of elements is observed:.. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The following trend in periodic properties of elements is observed:. 120 rows · periodic table trends:

You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom... Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance …

The following trend in periodic properties of elements is observed:.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in …

Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in …. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. 120 rows · periodic table trends: These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Atomic size decreases from left to right, because …

Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … 120 rows · periodic table trends:

These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom... The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 120 rows · periodic table trends: Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.

Atomic size decreases from left to right, because … . Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in …

Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in ….. .. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases ….. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.
Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.
119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … Atomic size decreases from left to right, because … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance …

Atomic size decreases from left to right, because … You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. 120 rows · periodic table trends: The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases …. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period... Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases …

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.. Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic size decreases from left to right, because … The following trend in periodic properties of elements is observed: These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. 120 rows · periodic table trends: 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom.
119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases …. The following trend in periodic properties of elements is observed: 120 rows · periodic table trends: These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. The following trend in periodic properties of elements is observed:

Nov 06, 2014 · the size of neutral atoms is drawn from the atomic radius, which is half the distance ….. Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. The following trend in periodic properties of elements is observed: 119 rows · feb 07, 2021 · the second atomic radius periodic trend is that atomic size decreases … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. These trends in atomic radii (as well as trends in various other chemical and physical properties of the elements) can be explained by considering the structure of the atom. Atomic size decreases from left to right, because … 120 rows · periodic table trends: You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row.

The following trend in periodic properties of elements is observed: Atom size trends the best way to understand atom size trends is by adding electrons, protons, and neutrons to an atom one by one to see how they affect atom size. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. You will learn why atom size gradually decreases from left to right across any given row in the periodic table, and increases again when you continue on to the next row. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The following trend in periodic properties of elements is observed: Atomic sizes (radii) the atomic size trends across a period and down a group ('family' in … 120 rows · periodic table trends: Mar 15, 2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.
